 +1 915 229 3004 (U.S.) |
+1 915 229 3004 (U.S.) |  +44 7452 242832 (U.K.)
+44 7452 242832 (U.K.)
 +1 915 229 3004 (U.S.) |
+1 915 229 3004 (U.S.) |  +44 7452 242832 (U.K.)
+44 7452 242832 (U.K.)

In 2020, the global Bioanalytical Testing Services Market was USD 1,298.3 Million and is expected to grow at a CAGR of 13.9% during the forecast period of 2022-2030.


Download Free Report Sample to learn more about this report.
In the past few years, biopharmaceutical companies have significantly outsourced resources that are essential for pre-clinical and clinical drug research and development to contract research organizations (CROs) to minimize costs, mitigate hazards, and achieve efficiencies. The drug development value chain relies heavily on bioanalytical testing to identify potential risks to safety and efficacy in both pre-clinical and clinical stages. The bioanalytical research during the drug development and lead optimization stage is performed in-house by the pharmaceuticals, while the later stages such as long-term toxicity and Phase II tests, are outsourced. This is mainly owing to the outsourcing of bioanalytical testing for final phases demands less method modifications, thereby lowering the cost of new process development by the CRO. Drug effectiveness and safety are major concerns for providing affordable therapies. Thus, CROs in this space occupies a remarkable position within the framework of drug development.
Bioanalytical testing of large molecules has been a complicated task for the pharmaceutical industry because the old techniques used for classical drugs cannot be adapted to large molecules with high molecular weight and complex structure. This has enabled the development of techniques such as Ligand Binding Assays (LBA), Maldi-TOF-MS, size exclusion affinity chromatography, HRMS etc. that can be used for bioanalytical large molecule testing. The CRO addresses the needs of the industry by introducing these services through mergers/acquisitions or expansions, which the pharmaceutical companies can leverage. For instance, Sartorius Stedim Biotech introduced a bioanalytical testing lab in the USA in November 2016. This specialized bioanalytical laboratory is designed to meet the growing demand for specialized assay platforms for the company's BioOutsource brand in North America and to promote the ongoing expansion of this unique service offering.
Bioanalytical Testing Services Market Driver:Increase in R&D Activities to Support the Market Growth
The increased focus of biopharmaceutical organizations on research and development services has created lucrative growth opportunities for bioanalytical testing services market growth in the future. Bioanalytical testing provides a quantitative measure of the active drugs and their metabolites in biological system for the purpose of pharmacokinetics/pharmacodynamics study. R&D investments are growing substantially because of the rising demand for drugs. Moreover, the availability of advanced analytical testing techniques such as hyphenated technique, kinetic method of analysis, electrochemical techniques enables specific monitoring of components in drug development. Development of such methods, however, requires extensive research.
Rising demand for bioanalytical testing services is attributed to the increasing prevalence of infectious diseases and other diseases such as HIV. Corona pandemic has transformed the current market scenario by creating a huge demand for these services. The demand for fast-acting medications to control chronic disorders has been anticipated over the prediction period. This is supported by rapid advances in research and development. Rapid biologics production speed up clinical tests, and it directly influences treatment options for cancer and other fatal diseases. Additionally, increasing application of bioanalytical tests in clinical trials of biomarkers, small molecules and biologics have supported the market growth. Development of biosimilar, combination molecules and other innovative medicines has resulted in an increase in demand for specific types of bioanalytical tests.
For instance, PPD Laboratories are. In this market from 30 years and have employed the most technologically advanced systems to develop assays and applied them to clinical trial samples across every stage of drug development. Their bioanalytical lab has supported more than 17 biosimilar programs in support of U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) submissions and has experience with more than 3,000 different biologics. Also, in Syneos Health, more than 80,000 biologics/biomarkers samples are analyzed annually. Recently in Feb 2020, Frontage has completed its Pennsylvania site expansion to boost its offering of bioanalytical services for late-stage cell and gene therapies. Their R&D facility for biologics now totals up to 80,000 squarefeet.
Bioanalytical Testing Services Market Restraint:High Investment Cost
Analytical testing services cost is still a concern for many small and medium-sized drug companies. The CRO model in the low-cost regions is considered the right model as long as they can deliver the desired quality of services. Volatile economic conditions in the bioanalytical testing services market often led to difficulty in budgetary management for small and medium-sized CROs. Moreover, regulatory operations have become a prominent challenge for several pharmaceutical firms, as most of these firms face the cost and labor pressure. Manufacturers struggle to obtain new product approvals, maintain compliance while ensuring the cost of competitive operation.
Also, due to the wide array of products available in the bioanalytical testing services market, pricing, positioning, and sales channel optimization play a crucial role in the success of a service or product. In developed and developing nations, there is a difference in price rates because of the variations in the purchasing capabilities and product availabilities. Furthermore, low return on investments and high cost of bringing new drug molecule to the market, that usually ranges in billions of dollars, will remain an uphill task for small and start-up CROs, that is expected to acts as a restraining factor for bioanalytical testing services market growth.
Bioanalytical Testing Services Market Recent Advancement:
Technological developments in medical laboratory services are expected to boost the demand for bioanalytical testing services in the coming years. Manufacturers plan to implement innovative and technically sophisticated clinical laboratory research technologies that will promote development in the industry. Moreover, attempts to eliminate new infectious diseases with the potential to cause serious harm, morbidity, and mortality will promote market development in analytical laboratory research. Moreover, the medical laboratory facilities seek to deliver more reliable, precise, effective health treatment and improved patient outcomes, thus encouraging development in the sector. Furthermore, collaborations and the implementation of innovative research devices for fast health evaluation coupled with innovation in R&D promote market growth. Increasing competition for bioanalytical testing research facilities would, therefore, fuel market growth in the years ahead.
In February 2020, Frontage Holdings Corporation, a contract research organization (“CRO”) known for its service covering science-driven, product development services, integrated, announced its expansion plan for bioanalytical laboratories in Exton, PA. The process would see its subsidiary Frontage Laboratories, Inc. assist. This would stimulate bioanalytical capabilities in the development of biomarkers, cell and gene therapy, biological and small molecular drugs, and high-throughput clinical sample management with a space of 10,000 sqft.

Download Free Report Sample to learn more about this report.
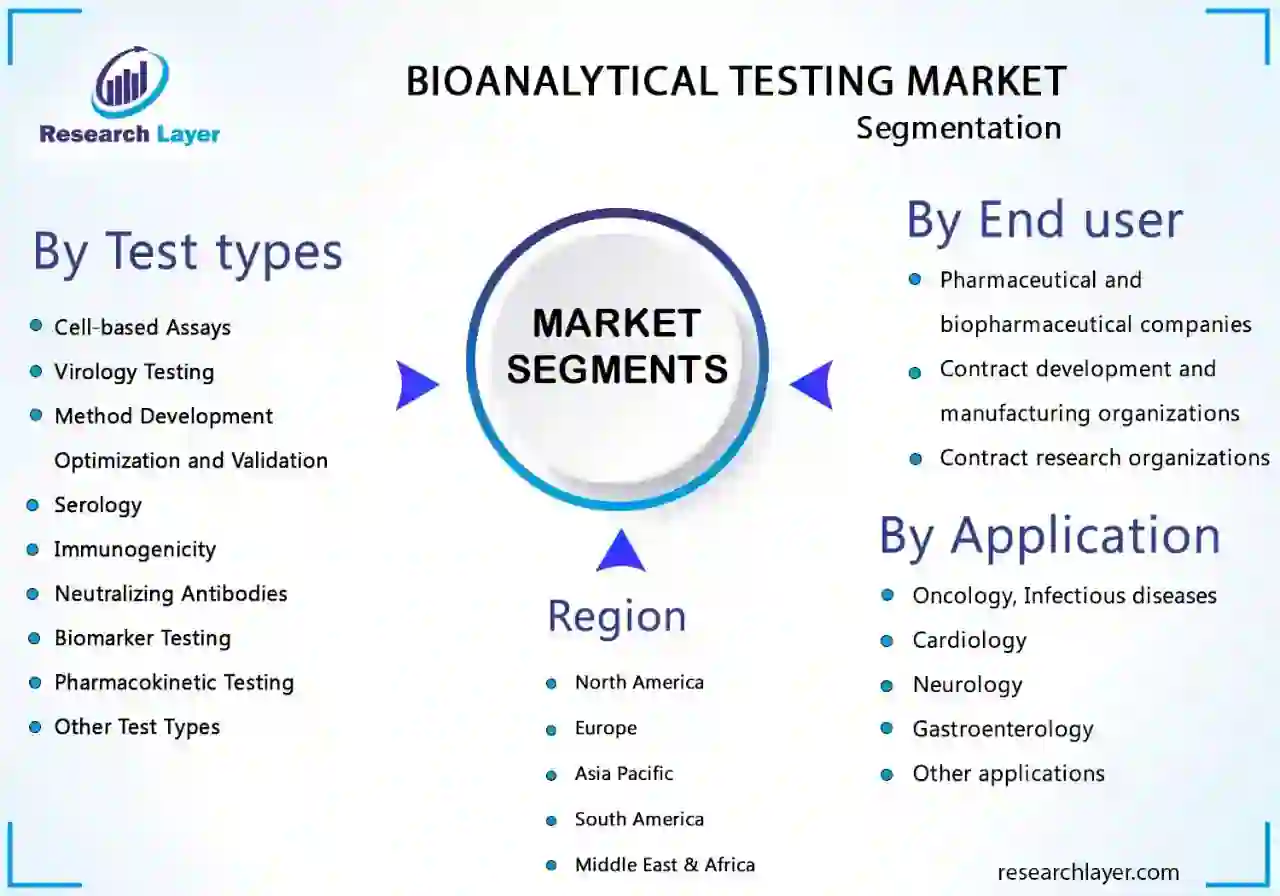
The bioanalytical testing services market is segmented into test type, application, and end user.
On the basis of test types, the bioanalytical testing services market is segmented into Cell-based Assays, Virology Testing, Method Development, Optimization and Validation, Serology, Immunogenicity, and Neutralizing, Antibodies, Biomarker Testing, Pharmacokinetic Testing, and Other Test Types.
Based on application, the bioanalytical testing services market is segmented into oncology, infectious diseases, cardiology, neurology, gastroenterology, and other applications.
On the basis of end user, the bioanalytical testing services market is segmented into pharmaceutical and biopharmaceutical companies, contract development and manufacturing organizations, and contract research organizations.
Based on application Oncology dominates the application segment of the bioanalytical testing services market. The vast proportion of the oncology segment can be attributed to the mounting number of clinical trials and the increased occurrence of cancer round the globe. As per the WHO, cancer is the foremost cause of mortality globally, which accounted for nearly 9.6 million deaths in 2018. Thus, with increasing cancer burden coupled with numerous risk factors including obesity, unhealthy lifestyle, hormone replacement therapy, among others, the demand for cancer tests will rise significantly in the years ahead, translating to bioanalytical testing services market growth.
Clinical cancer is going through an exciting change at the moment. Historically, cancer treatment has been comprised of surgery, chemotherapy, and radiotherapy, but lately, novel treatments have emerged as promising tools. Antibody-drug conjugates (ADCs), for example, use high sensitivity and specificity to bring a tumor-killing agent to its location only to eradicate tumor cells, leaving healthy tissue untouchable. Additionally, immuno-oncological strategies are being developed that leverage the patients' own immune systems to kill cancer cells that possess immense potential. Such molecular and probably customized methods will lead to the dissolution of conventional chemotherapies and a new standard of care for curative cancer and perhaps even to the eradication of certain cancers.
Based on region, the global bioanalytical testing services market is segmented into North America, Europe, Asia Pacific, Central, South America and Caribbean, and the Middle East & Africa.
The North America bioanalytical testing service market will show a rapid growth trend and dominate the market in terms of revenue during the forecast study period. The growth can be attributable to being one of the top manufacturing centers for highly effective, complex, and high-end pharmaceutical products, and a large presence of prominent players operating in the bioanalytical testing services market.
· In February 2020, Frontage opened a new bioanalytical laboratory in Exton, PA, through its wholly-owned subsidiary, Frontage Laboratories, Inc. The newly developed facility covers a lab space of around 10,000 square feet. It will increase bioanalytical potentials in biologics, biomarkers, drug development, and high-throughput clinical sample management.
· In January 2020, Charles River collaborated with Fios Genomics (the U.K.). This corporation helped the firm in the outsourcing and analysis of high-dimensional, multi-variant datasets related to drug development.
· In May 2019, Thermo Fisher Scientific launched immune cell types assay named as ‘Applied Biosystems PureQuant Assays’. The launch will help the firm in expanding its product portfolio for identification and purity testing of cell-based medications.
· In April 2019, PerkinElmer acquired Cisbio Bioassays.
· In March 2019, Guardant Health acquired Bellwether Bio, a new company engaged in the development of next-generation oncology diagnosis using cell-free DNA (liquid biopsy). The acquisition assisted the firm to strengthen its cancer diagnostics product portfolio through novel technologies.
· In January 2019, Danaher launched its ClearLLab 10C System for clinical Flow Cytometry labs. The new system comprises the 1st 10-color CE-IVD panels of immunophenotyping reagents for lymphoid and myeloid lineages.
· In July 2018, Biodesix, Inc., a leading player operating in molecular diagnostics, acquired Integrated Diagnostics, Inc. The acquisition involved Inid’s XL2 test, that is its foremost non-invasive oncology test for screening lung cancer, thus offering Biodesix a competitive gain and expanding its product portfolio.
Moreover, high research and development expenditure by the pharmaceutical companies and rising outsourcing activities in this region will promote the regional market expansion.
In North America, the U.S. holds a significant market share, owing to increased patient pool suffering from chronic diseases and raised adoption of the peptides, other large molecule therapeutics as an alternative for small molecules that have more side effects. For instance, as per the International Diabetes Federation in 2019, about 48 million adults in North America were living with diabetes and are projected to continue resulting in higher demand for bioanalysis of novel (large molecule compounds) peptide therapeutics such as dipeptidyl peptidase 4 (DPP-4) inhibitors, glucagon-like peptide 1(GLP-1) analogs to limit the growing volume of patient pool with Type 2 diabetes, thus driving the market in the region, contributing its highest revenue share during the forecast period.
Some of the major key players in the bioanalytical testing services market are Medpace, IQVIA, SGS SA, Charles River Laboratories, Covance Inc. (Laboratory Corporation Of America Holdings), WuXi AppTec, Eurofins Scientific, Intertek Group plc, Pra Health Sciences, Syneos Health, and LGC Limited.
In 2019, Eurofins Scientific acquired Transplant Genomics Inc. (US) to expand its transplantation bioanalytical testing footprint.
Moreover, in same year Frontage lab acquired RMI Pharmacokinetics a CRO located in North Wales, Pennsylvania. RMI offers a variety of services including full range of metabolite profiling and early stage development analysis, late discovery cross species comparison, and pre-clinical animal radiolabeled mass spectrometric bioanalysis. The RMI acquisition will enhance the company's DMPK capacity with additional scientists, equipment, and facilities to be used in the provision of existing and novel services to the customers, and effectively expand the current client base of Frontage lab.
in 2019, Frontage lab has enhanced the capabilities and scale of DMPK and bioanalytical services and a geographic foothold into Canada and the west coast of North America with the acquisition of BRI Biopharmaceutical Research Inc. a CRO that was established more than 20 years ago in Vancouver, Canada.
This report categorizes the Bioanalytical Testing Services Market as follows:
In 2020, the global bioanalytical testing services market was USD 1,298.3 Million and is expected to grow at the CAGR of 13.9% during the forecast period of 2022-2030.
Expected CAGR for the Bioanalytical Testing Services Market is 13.9%.
Key players in the global Bioanalytical Testing Services Market are Medpace, IQVIA, SGS SA, Charles River Laboratories, Covance Inc. (Laboratory Corporation Of America Holdings), WuXi AppTec, Eurofins Scientific, Intertek Group plc, Pra Health Sciences, Syneos Health, LGC Limited.
Following Segments Coverd in Bioanalytical Testing Services Market are Test types, End user, Application and Region.


Report Code :
RL6519
Published on :
Nov 2022
Request a Free Sample Report